Abstract

Introduction The CARAVAGGIO trial was a multinational, randomised, investigator initiated, open-label with blind outcome assessment, noninferiority trial which demonstrated noninferiority of apixaban to dalteparin for treatment of cancer related venous thromboembolism (VTE) without an increased risk of major bleeding. In patients with acute VTE, the rates of recurrence and major bleeding are highest during the first weeks of anticoagulation (1).
We sought to evaluate the efficacy and safety of apixaban during the initial high-risk phase of treatment in patients with cancer associated VTE. Specifically, we performed a pre-specified sub-analysis of the time courses of recurrence and bleeding, with the aim to determine the incidence after 7, 30 and 90 days of treatment with apixaban compared with dalteparin.
Methods The study design and methods of the CARAVAGGIO trial (CARAVAGGIO; NCT03045406; https://clinicaltrials.gov/ct2/show/NCT03045406) have been described (2). Briefly, CARAVAGGIO compared the efficacy and safety of apixaban with dalteparin in consenting patients who had active cancer and a newly diagnosed symptomatic or incidental proximal lower-limb deep-vein thrombosis (DVT) or pulmonary embolism (PE).
Eligible patients were randomly assigned to receive monotherapy with either apixaban or dalteparin for 6 months. The primary efficacy outcome was the incidence of objectively confirmed VTE. The primary safety outcome was major and clinically relevant non-major (CRNM) bleeding.
Results The CARAVAGGIO study enrolled 1170 patients from 119 centres in eleven countries from April 2017 through June 2019); 1155 patients were included in the modified intention-to-treat analysis. The demographic and clinical characteristics of the patients were similar in the two treatment groups.
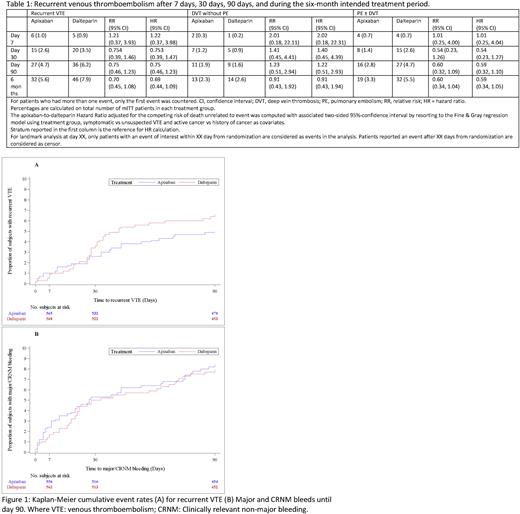
Of the 78 recurrent VTE events 11 (14.1%) occurred in the first 7 days, 35 (44.8%) in the first 30 days and 63 (80.7%) in the first 90 days. Recurrent events occurred in 6 (1.0%) in the apixaban group and 5 (0.9%) in the dalteparin group (RR 1.21; 95% CI 0.37-3.93) by day 7, 15 (2.6%) in the apixaban group and 20 (3.5%) in the dalteparin group (RR 0.75; 95% CI 0.39-1.46) by day 30, and in 27 (4.7%) in the apixaban group and 36 (6.2%) in the dalteparin group (RR 0.75; 95% CI 0.46-1.23) by day 90. By 6 months, recurrent events occurred in 32 (5.6%) in the apixaban group and 46 (7.9%) in the dalteparin group (RR 0.70; 95% CI 0.45-1.08). (Table 1).
During the six months of treatment, major and CRNM bleeding occurred in 70 patients who received apixaban (12.2%) and in 56 patients who received dalteparin (9.7%) (RR 1.26; 95% CI 0.90-1.75). Of the total 126 bleeding events, 24 (19.0%) occurred during the first 7 days; 58 (46.0%) during the first 30 days, and 89 (70.6%) during the first 90 days. Bleeding events occurred in 14 (2.4%) in the apixaban group and 10 (1.7%) in the dalteparin group (RR 1.40; 95% CI 0.63-3.14) by day 7, 30 (5.2%) in the apixaban group and 28 (4.8%) in the dalteparin group (RR 1.08; 95% CI 0.65-1.78) by day 30, and in 46 (8.0%) in the apixaban group and 43 (7.4%) in the dalteparin group (RR 1.07; 95% CI 0.72-1.60) by day 90. The Kaplan-Meier plot of time to recurrent VTE and major and CRNM bleeding is shown in figure 1.
Conclusion The results of this sub-analysis of the CARAVAGGIO trial demonstrate no difference of oral apixaban to subcutaneous dalteparin at all time points. Specifically, there was no increase in recurrent VTE or bleeding events at 7 days, 30 days, 90 days and 6 months. This supports early initiation of oral anticoagulation. Oral versus subcutaneous anticoagulation has the advantage of ease of administration and outpatient delivery which may improve patients’ quality of life - an important consideration in patients with cancer.
Reference 1. Raskob GE, Gallus AS, Sanders P, Thompson JR, Agnelli G, Buller HR, et al. Early time courses of recurrent thromboembolism and bleeding during apixaban or enoxaparin/warfarin therapy. A sub-analysis of the AMPLIFY trial. Thrombosis and haemostasis. 2016;115(4):809-16.
2. Agnelli G, Becattini C, Meyer G, Muñoz A, Huisman MV, Connors JM, et al. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. New England Journal of Medicine. 2020;382(17):1599-607.
Disclosures
Cohen:Alexion/Astra Zeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bayer AG: Consultancy, Honoraria, Speakers Bureau; BMS/Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau. Alikhan:Boehringer Ingelheim: Consultancy; Daiichi Sankyo: Consultancy; Pfizer: Consultancy; BMS: Consultancy; Bayer: Consultancy. Connors:Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Roche: Honoraria, Speakers Bureau; CSL Behring: Research Funding; Werfen: Membership on an entity's Board of Directors or advisory committees; Anthos: Membership on an entity's Board of Directors or advisory committees; Abbott: Consultancy, Membership on an entity's Board of Directors or advisory committees; Alnylam: Consultancy. Munoz:Genincode biotech company: Patents & Royalties: Risk assessment model in venous thromboembolism in cancer patients; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses/accommodations, Research Funding; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses/accommodations; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Leo Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees; Lilly: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses/accommodations; Servier: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Rovi: Research Funding, Speakers Bureau; Bayer: Speakers Bureau; Menarini: Speakers Bureau; STADA: Speakers Bureau; Merck Serono: Other: Travel expenses/accommodations; Amgen: Other: Travel expenses/accommodations. Bauersachs:Pfizer: Consultancy, Honoraria; Viatris: Consultancy, Honoraria; LEO: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Bristol Meyers Squibb: Consultancy, Honoraria. Becattini:Pfizer: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Bayer AG: Consultancy, Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.